Monitoring post-traumatic osteoarthritis progression for early intervention
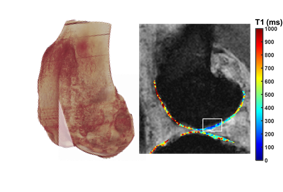
In the developed world, osteoarthritis (OA) is the most common articular skeletal disease, and there is no truly beneficial treatment available which can halt or reverse degeneration. Treatments focus on symptom management rather than stopping the disorder. OA is a well-described, chronic joint condition with clinical manifestations including substantial synovial joint pain, swelling, and stiffness. Despite the promising candidates to slow, stop, or reverse OA, the turnover of synovial fluid in the joint space quickly removes drug from the joint space such that there is no longer efficacious activity. In this project, we use ultra-high field 7T MRI and micro-Computed Tomography for quantitative PTOA detection and monitoring in a rabbit model and are using nanotechnological approaches to administer therapeutics directly, locally, and in a sustained manner, to promote and enhance healing.

Plasmonic nanoparticles for sensing and theranostics
Noble metal nanoparticles exhibit unique and tunable optical properties on account of their surface plasmon resonance. Metallic nanoparticles can provide therapeutic and diagnostic advantages that can be used in conjunction with cargo delivery, such as highly localized photothermal heating responses, enhanced contrast proprieties for imaging, and they can act as molecular sensors. Our lab has used metallic nanoparticles as radiosensitizers in lung cancer cell lines, for targeted intratumoral delivery in a murine model of non-small cell lung cancer, and to detect important serum and tissue diagnostic markers with high sensitivity.
Controlled release of osteogenic factors for bone induction
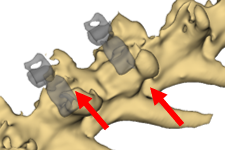
Spinal fusion is one of the most common surgeries performed in the neck or back and, along with large segmental defects, remains one of the toughest bone-healing applications. The procedure’s frequency has significantly increased over the last 15 years, concurrent with the increasing popularity and use of osteobiologics to improve fusion. Accelerated bone formation and enhanced fracture healing provide valuable treatment methods as they can reduce healing time, increase the quality of bone generated, and improve patient outcomes. This project entails the design of a spinal fusion implant permitting sustained release of osteogenic growth peptide (OGP) through a nanochannel membrane in an established large animal (rabbit) model.
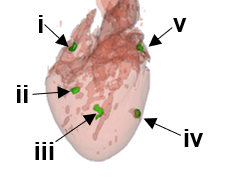
Hydrogel delivery of bioengineered materials for prolonged retention in deep target organs
Delivery of cells, drugs, genes, and other bioengineered materials vis scaffolds are advantageous for achieving high loading and efficiency to specific sites. However, placement of such scaffolds into deep tissues is achieved by implantation directly into the target site, with open surgical intervention. In this project, we are testing ways to deliver bioengineered nanomaterials in an alginate scaffold into porcine cardiac tissue. Use of radio-opaque bioengineered materials allows us to visualize placement, measure injection volumes and study gel degradation along with material dissipation into the systemic circulation.
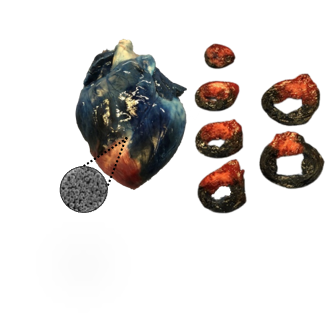
Controlled intrapericardial delivery of prostaglandin formulations for heart failure
Currently, in the majority of cardiac drug delivery systems, only a small fraction of the therapeutic agents reach and remain in the heart for an extended period of time because of coronary washout. Our innovation lies in the intrapericardial delivery of poly(d,l-lactide-co-glycolide) nanoparticles (PLGA-NPs) encapsulated with prostaglandin analogs. Prostaglandins stimulate the production of endogenous hepatocyte growth factor (HGF) via the cAMP pathway. HGF is cardioprotective and increased levels in patients with acute myocardial infarction have improved left ventricular function. We utilize local intrapericardial delivery of drug eluting PLGA-NPs. Our engineered nanoparticles and delivery methodology allow for controlled, sustained, and local targeted delivery of a safe drug with both regenerative and protective benefits.